
Tepezza® infusions Linked to Hearing Loss and tinnitus
1-800-LAW-FIRM is reviewing claims that consumers who were prescribed Tepezza® infusions to treat Thyroid Eye Disease (TED), caused by hyperthyroidism and linked to Graves' Disease, experienced hearing issues. Lawsuits claim that the manufacturer of Tepezza®, Horizon Pharmaceuticals Inc., failed to adequately warn users and the medical community about hearing problems that surfaced during clinical trials.
If you or a loved one experienced hearing issues after receiving Tepezza® infusions you may be able to hold the manufacturer accountable. Schedule a no-obligation, free claim review with a member of our team by filling out the following form.
According to lawsuits filed against Horizon Pharmaceuticals Inc., the pharmaceutical company was aware of the severe health risks Tepezza® posed to consumers who used the drug to treat Thyroid Eye Disease (TED). The lawsuits focus on the manufacturer's failure to warn doctors or patients of those risks in its disclaimers.
FREE CLAIM REVIEW
Tepezza® was granted Orphan Drug designation when approved in January 2020, which allowed the medication to be introduced without extensive testing since there is no other drug treatment available for Thyroid Eye Disease. However, Tepezza® clinical trials have been criticized since the studies only involved 84 patients, which may not have accurately portrayed all potential side effects or their severity. Even though the FDA approved the special designation, drug makers still have a duty to ensure the safety of the medication they sell and issue warning labels about the potential side effects.
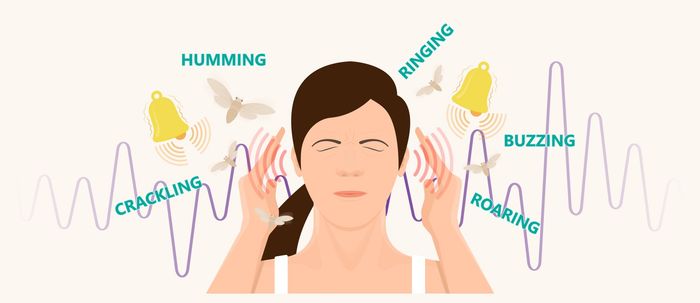
A 2021 study published by The Endocrine Society found as many as 65% of consumers that were prescribed Tepezza® during the short time it has been on the market experienced the following hearing problems at an alarming rate:
- Hearing loss
- Ringing in the ears (Tinnitus)
- Difficulty hearing
- Ear plugging sensation
- Clicking and popping sounds
- Muffled sounds
- Deafness
- Sensitivity to certain sounds
According to allegations raised in Tepezza® lawsuits, Horizon Pharmaceuticals reported to the FDA that the hearing loss side effects from Tepezza® were only temporary, suggesting that the problems would resolve after months of stopping infusion treatments. However, medical journals have published case studies documenting no improvements in hearing loss in the months after receiving Tepezza® infusions.
Despite these known risks, Horizon Pharmaceuticals failed to adequately warn that Tepezza® may cause hearing loss or instruct patients and doctors about the importance of regular audiological testing to detect hearing problems after taking Tepezza®.
WHAT COULD A TEPEZZA® VICTIM RECOVER AS PART OF THE LITIGATION?
Suppose hearing loss warnings and instructions about the importance of monitoring for hearing problems after receiving Tepezza® infusions had been provided. In that case, consumers might have avoided permanent and disabling injuries.
As a result of Horizon Pharmaceuticals’ apparent decision to place a desire for profits before the health and safety of consumers, Tepezza® hearing loss settlements may be available. Ultimately, the amount each plaintiff may settle for depends on their specific case and the extent of their injuries.
Tepezza® victims may be able to recover the following economic and non-economic damages from Horizon Pharmaceuticals Inc.
- Past and future anticipated costs of medical treatment and other medical expenses
- Loss of past and future earnings from being too ill to work
- Pain and suffering, including mental distress and losing quality of life
Since the litigation remains in the early stages, with the first Tepezza® lawsuits filed in late 2022, there have not been any global Tepezza® settlements or lawsuit payouts. However, as the cases progress, the manufacturer is expected to engage in Tepezza® hearing loss settlement talks to resolve claims.